By Nathan
Newly created “micro-batteries” that are only a few millimeters in size are now the most powerful batteries in the world. The new batteries, created by researchers at the University of Illinois, greatly out-power “even the best supercapacitors,” while being only a fraction of their size.
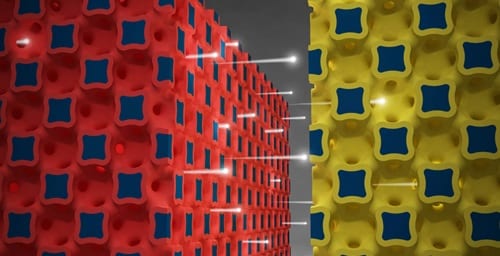
“The graphic illustrates a high power battery technology from the University of Illinois. Ions flow between three-dimensional micro-electrodes in a lithium ion battery.”
“They pack such a punch that a driver could use a cellphone powered by these batteries to jump-start a dead car battery – and then recharge the phone in the blink of an eye,” a University of Illinois press release put out yesterday noted.
Sounds like a potentially significant technological improvement. Such batteries could certainly have a use in electric vehicles, and as a means of renewable energy storage, if they can be produced cheaply enough.
“This is a whole new way to think about batteries,” said William P. King, University of Illinois professor of mechanical science and engineering. “A battery can deliver far more power than anybody ever thought. In recent decades, electronics have gotten small. The thinking parts of computers have gotten small. And the battery has lagged far behind. This is a microtechnology that could change all of that. Now the power source is as high-performance as the rest of it.”
What makes this new technology sound interesting though isn’t simply the increased power, it’s the potential for simultaneously possessing high power transmission and high energy storage. As of now, there’s a trade-off forced by technological limitations — it’s either one or the other, not both.
“There’s a sacrifice,” said James Pikul. “If you want high energy you can’t get high power; if you want high power it’s very difficult to get high energy. But for very interesting applications, especially modern applications, you really need both. That’s what our batteries are starting to do. We’re really pushing into an area in the energy storage design space that is not currently available with technologies today.”
Some of the potential uses are certainly interesting: electronic devices as much as 30 times smaller, credit-card-thin cell phones that can recharge in a second, high-power lasers, portable high-power medical devices, etc.
What makes these batteries so much better than others? How did the researchers do it? I’ll let the University explain:
“The batteries owe their high performance to their internal three-dimensional microstructure. Batteries have two key components: the anode (minus side) and cathode (plus side). Building on a novel fast-charging cathode design by materials science and engineering professor Paul Braun’s group, King and Pikul developed a matching anode and then developed a new way to integrate the two components at the microscale to make a complete battery with superior performance.”
The researchers indicate that the batteries are indeed rechargeable and that they can charge approximately 1,000 times faster than competing technologies. That’s no incremental improvement, but we’ll see if they can bring the technology to market.
The researchers are currently working on developing a low-cost manufacturing paradigm for the technology.
The new technology is outlined in the April 16 issue of Nature Communications.
This article was originally published on CleanTechnica. Reproduced with permission