Swiss researchers have published a study they believe could be “central” to the large-scale commercialisation of perovskite-based solar cells, and promised a solution to the super efficient technology’s hazardous chemical problem.
Perovskites – inexpensive synthetic materials that are relatively simple to produce – hold huge promise in the world of solar cell research for their inherent flexibility and super high efficiencies of energy conversion.
On the flip-side, the most common perovskites used in photovoltaics contain lead, or methylammonium lead halide, the water solubility of which can become a serious environmental and human health hazard if the panel fails and the lead leaches out into the soil.
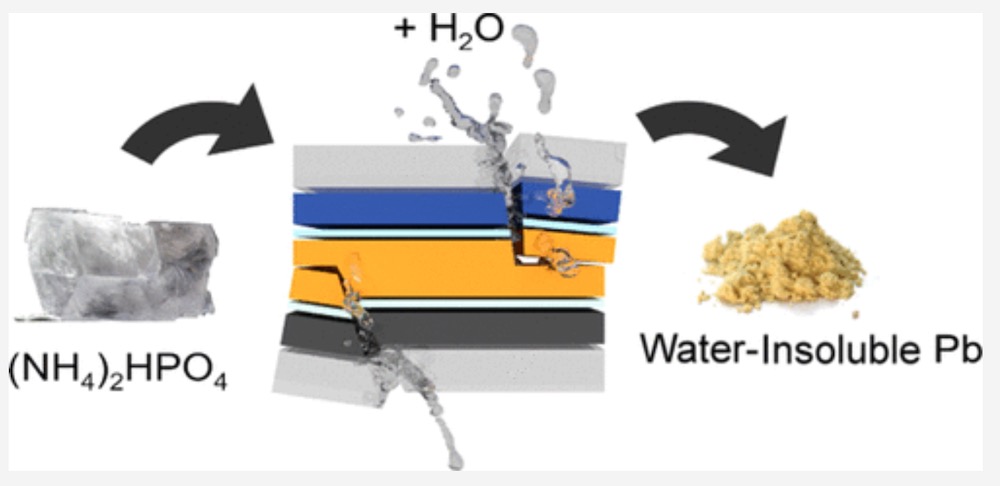
A team at the Ecole Polytechnique Fédérale de Lausanne (or Swiss Federal Institute of Technology) has come up a possible solution to this, using a transparent phosphate salt that doesn’t compromise the solar cells’ performance but prevents the lead from getting into the environment.
According to Science Daily, the solution works so that, if the solar panel fails, the phosphate salt immediately reacts with the lead to produce a water-insoluble compound that cannot leach out to the soil, and which can be recycled.
“The solar energy-to-electricity conversion of perovskite solar cells is unbelievably high, around 25%, which is now approaching the performance of the best silicon solar cells,” said Professor László Forró at EPFL’s School of Basic Sciences.
“But their central element is lead, which is a poison; if the solar panel fails, it can wash out into the soil, get into the food chain, and cause serious diseases.”
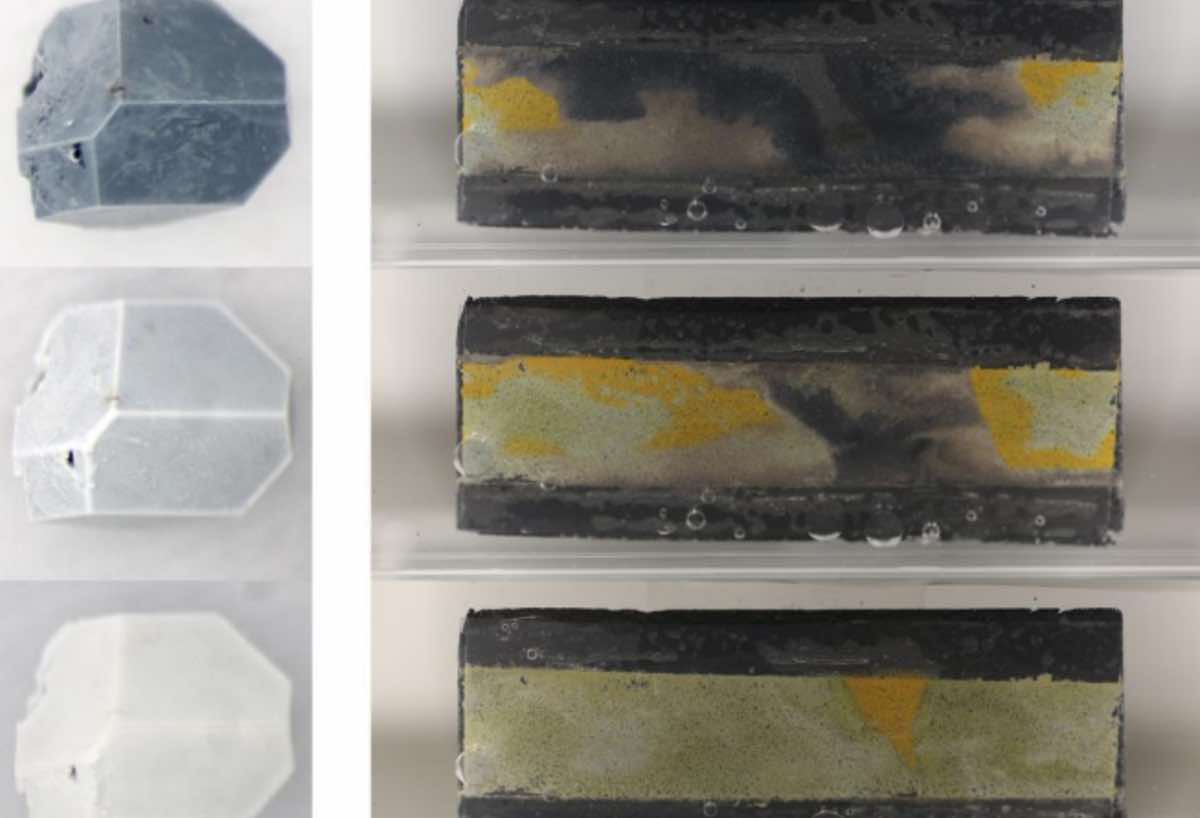
Endre Horváth, the study’s first author, had first discovered “a few years ago” that cheap and transparent phosphate salt crystals, like those found in soil fertilizers, could be incorporated into the sandwich-like lead halide perovskite devices, like photodetectors, LEDs or solar cells.
“These salts instantaneously react with lead ions in the presence of water, and precipitate them into extremely non-water-soluble lead phosphates,” Horváth said.
“The ‘fail-safe’ chemistry keeps lead ions from leaching out and can render perovskite devices safer to use in the environment or close to humans,” added Márton Kollár, the chemist behind the growth of perovskite crystals.
Pavao Andrievic, who characterised the sensitive photodetectors, said the team suggested that the broad community of researchers and R&D centres working on devices like solar cells implemented the approach in their respective prototypes.
Tthe study is expected to stimulate research, enabling lead halide perovskite solar cells to reach a similar environmental risk category as the commercially available, nonwater-soluble heavy metal-containing CdTe and gallium diselenide technologies.
“This is an extremely important study — I would say, a central one — for large-scale commercialisation of perovskite-based solar cells,” Forró added.
The study was published in the journal ACS Applied Materials & Interfaces.