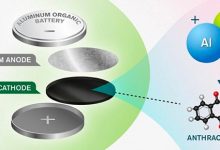
European researchers have developed a concept for a new aluminium battery which could boasts twice the energy density of previous versions and could be made of abundant materials, resulting in a battery which could reduce production costs as well as its impact on the environment.
Researchers from Chalmers University of Technology, Sweden, and the National Institute of Chemistry made the announcement of their concept development at the end of September, and published details of their work in the journal Energy Storage Materials.
Instead of using graphite as the cathode (the positive electrode) the researchers have replaced it with an organic nanostructured cathode which has been made of the carbon-based molecule anthraquinone.
“The material costs and environmental impacts that we envisage from our new concept are much lower than what we see today, making them feasible for large scale usage, such as solar cell parks, or storage of wind energy, for example,” said Patrik Johansson, Professor at the Department of Physics at Chalmers.
“Additionally, our new battery concept has twice the energy density compared with the aluminium batteries that are ‘state of the art’ today.”
Extensively developed by Jan Bitenc, previously a guest researcher at Chalmers from the group at the National Institute of Chemistry in Slovenia, anthraquinone enables storage of positive charge-carriers from the electrolyte – the solution in which ions move between the electrodes – in turn making possible higher energy density in the battery.
Previous aluminium batteries have failed to compete as the graphite used in these batteries provided too low energy content to create battery cells with enough performance to be of any use.
Swapping out the graphite for anthraquinone, however, increases the likelihood of aluminium batteries being able to compete in an already developed market. Further, aluminium batteries would be able to make use of the fact that there already exists an established industry for the manufacturing and recycling of its main component, ie, aluminium.
“Because the new cathode material makes it possible to use a more appropriate charge-carrier, the batteries can make better usage of aluminium’s potential,” added Chalmers researcher Niklas Lindahl, who studies the internal mechanisms which govern energy storage.
“Now, we are continuing the work by looking for an even better electrolyte. The current version contains chlorine — we want to get rid of that.”
“Of course, we hope that they can” compete and eventually replace lithium-ion batteries, said Patrik Johansson.
“But above all, they can be complementary, ensuring that lithium-ion batteries are only used where strictly necessary. So far, aluminium batteries are only half as energy dense as lithium-ion batteries, but our long-term goal is to achieve the same energy density.
“There remains work to do with the electrolyte, and with developing better charging mechanisms, but aluminium is in principle a significantly better charge carrier than lithium, since it is multivalent — which means every ion ‘compensates’ for several electrons. Furthermore, the batteries have the potential to be significantly less environmentally harmful.”